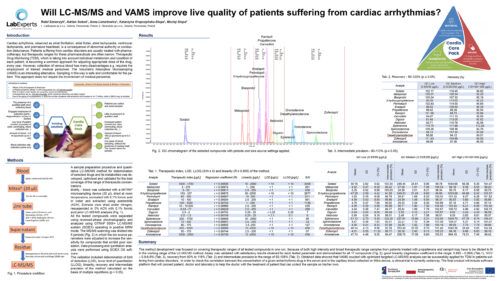
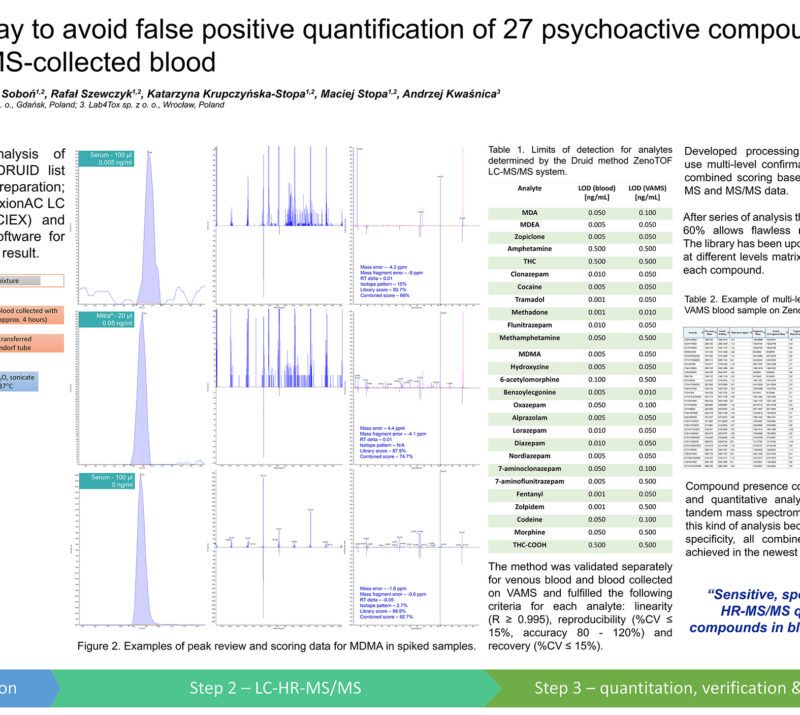
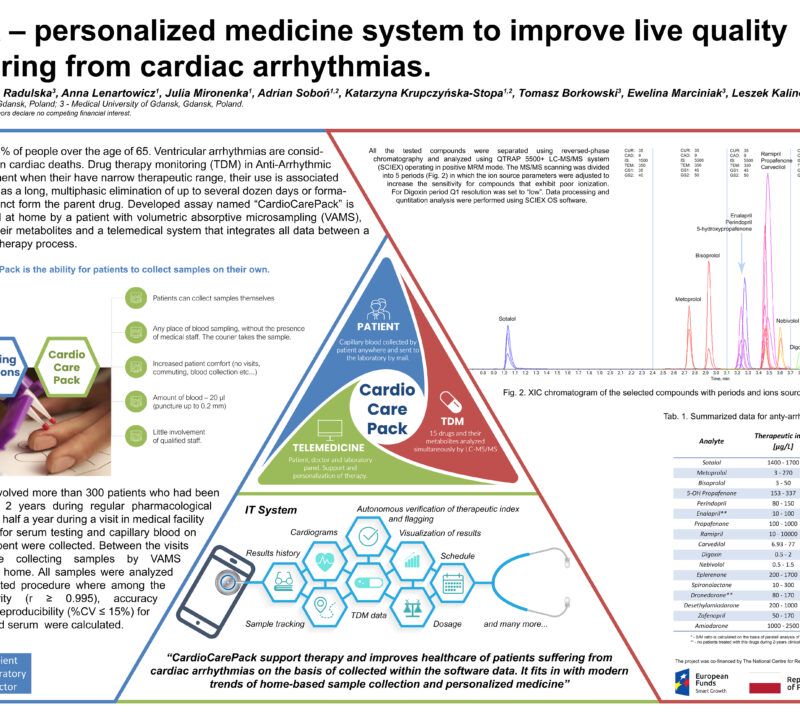
Will LC-MS/MS and VAMS improve live quality of patients suffering from cardiac arrhythmias?Rafał Szewczyk1, 2; Anna Lenartowicz1; Adrian Soboń1, 2; Katarzyna Krupczyńska-Stopa1, 2; Maciej Stopa1, 2 1LabExperts Sp. z o.o., Gdansk, Poland; 2Bioanalytic Sp. z o.o., Gdansk, Poland; AbstractCardiac arrhythmia, observed as atrial fibrillation, atrial flutter, atrial tachycardia, ventricular tachycardia, and premature heartbeat, is a consequence of abnormal authority or conduction disturbance. Patients suffering from cardiac disorders are usually treated with pharmacotherapy, but therapeutic ranges for these pharmaceuticals are often narrow. Therapeutic Drug Monitoring (TDM), which is taking into account individual metabolism and condition of each patient, is becoming a common approach for adjusting appropriate dose of the drug, every year. However, collection of venous blood has many disadvantages e.g. requires the employment of trained medical personnel. The Volumetric Absorptive Microsampling (VAMS) is an interesting alternative. Sampling in this way is safe and comfortable for the patient. This approach does not require the involvement of medical personnel. Methods:Blood was collected with a MITRA® microsampling device (20 µl), dried for 24h at room temperature, sonicated with 0.1% formic acid in water and extracted using acetonitrile. A quantitative LC-MS/MS analysis method of 15 antiarrhythmic drugs and 2 metabolites was developed and validated. All the tested compounds were separated using reversed-phase chromatography and analyzed using mass spectrometer QTRAP 5500+ (Sciex) operating in positive MRM mode. The MS/MS scanning was divided into 5 periods in which the ion source parameters were adjusted to increase the sensitivity for compounds that exhibit poor ionization. Data processing were performed using Sciex OS software. Preliminary Data:First stage of the project was focused on development of a single assay for quantitative determination of 15 antiarrhythmic drugs (sotalol, metoprolol, bisoprolol, propafenone, carvedilol, nebivolol, dronedarone, amiodarone, digoxin, perindopril, enalapril, ramipril, eplerenone, spironolactone, zofenopril) and 2 metabolites (5-OH propafenone and desethylamiodarone) at therapeutically relevant levels using only 20 µL of blood collected using finger-prick method and a Mitra® device. The method development was focused on covering therapeutic ranges of all tested compounds in one run. Because of both high intensity and broad therapeutic range samples from patients treated with propafenone and ramipril may have to be diluted to fit in the working range of the LC-MS/MS method. Assay was validated with satisfactory results obtained for each tested parameter and demonstrated for all 17 compounds: good linearity (regression coefficient in the range: 0.995 – 0.999), %CV – 0.9-6.6%, recovery from 80% to 116% and intermediate precision in the range of 92-108%. Obtained data showed that VAMS coupled with optimized targeted LC-MS/MS analysis can be successfully applied for TDM in patients suffering from cardiac disorders. In order to check the correlation between the concentration of a given antiarrhythmic drug in the serum and in the capillary blood collected on Mitra device, a clinical trial is currently underway. The final product will include software platform that will connect patient, doctor and laboratory to help the doctor with the treatment of patient that can collect the sample on his/her own. Novel aspect:New diagnostic procedure using VAMS and LC-MS/MS determination of 17 antiarrhythmic drugs and their metabolites commonly used in Central Europe. |
ASMS 2021, Philadelphia, USA, 31.10 – 04.11, 2021 (online). |