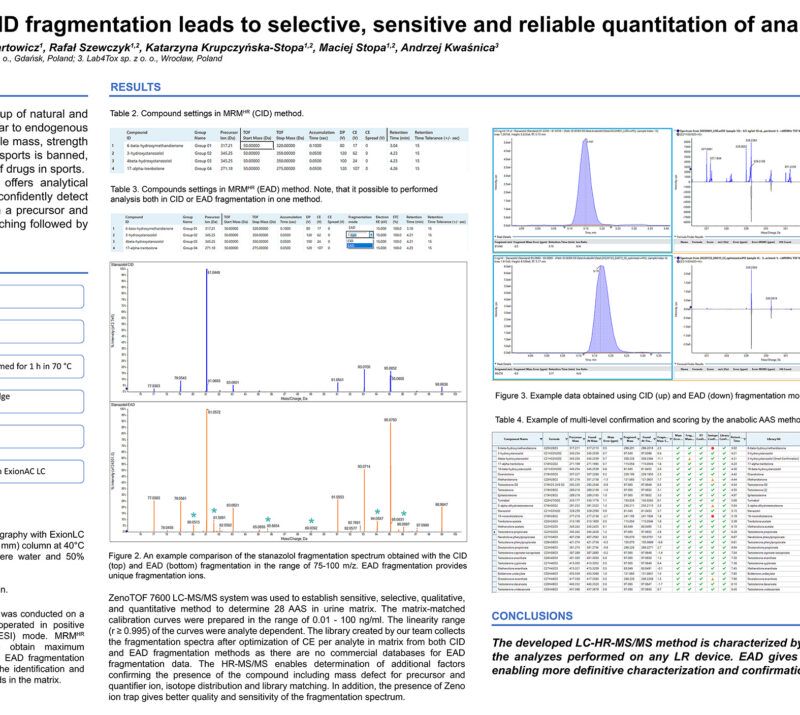
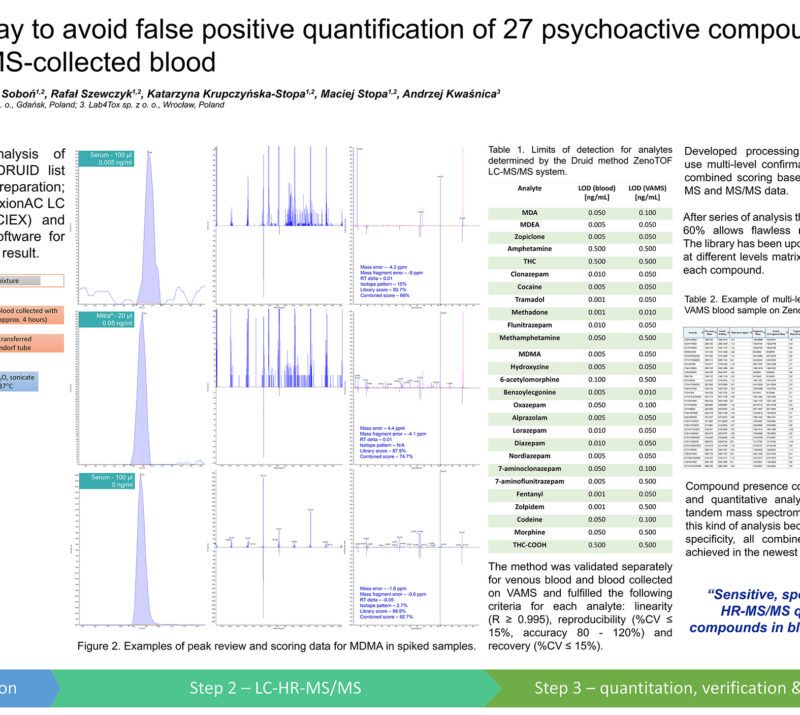
LC-MS/MS and VAMS a well-coordinated team for toxicological analyzes.Rafał Szewczyk (1,2); Anna Lenartowicz (1); Julia Mironenka (1); Adrian Soboń (1,2); Katarzyna Krupczyńska-Stopa (1,2); Maciej Stopa (1,2); Andrzej Kwaśnica (3) AbstractIntroductionAccurate collection of a blood sample at the scene of an incident and its reliable analysis may seem like a challenging task. The classic venous blood collection has many disadvantages e.g. requires a place prepared for this purpose, trained medical person, an adequate protection and transport of the sample. The Volumetric Absorptive Microsampling (VAMS) is an interesting alternative. Sampling in this way is safe, quick and convenience – a blood sample is taken from the finger. This can be done by a non-medical professional. Samples are safe during shipping and do not require controlled conditions before analysis which is the task of liquid chromatography-mass spectrometry. This well-coordinated team accelerates, simplifies and increases availability with parallel lowering the cost of analysis. Methods:Blood was collected with a MITRA® microsampling device (20 µl), dried for 24h at room temperature, sonicated with 0.1% formic acid in water and extracted using methanol:acetonitrile (1:1) with 0.1% formic acid. A quantitative LC-MS/MS analysis method of 27 psychoactive compounds and 5 internal standards was developed and validated. All the tested compounds were separated using reversed-phase chromatography and analyzed using mass spectrometer QTRAP 5500+ (SCIEX) operating in positive and negative scheduled MRM mode. 2 MRM pairs (quantifier and qualifier ions) per tested compound and one for internal standard were used. Data processing were performed using SCIEX OS software. Preliminary Data:First step of the method development was focused on optimization of LC-MS/MS parameters to cover all 27 analytes (6-acetylmorphine, 7-aminoflunitrazepam, 7-aminoclonazepam, alprazolam, amphetamine, benzoylecgonine, diazepam, phentanyl, flunitrazepam, hydroxyzine, clonazepam, codeine, cocaine, lorazepam, MDA, MDEA, MDMA, methadone, methamphetamine, morphine, nordiazepam, oxazepam, THC, THC-COOH, tramadol, zolpidem, zopiclone) in one selective, sensitive and linear assay for quantitative determination that fulfills the requirements of WADA guidelines at relevant levels using only 20 µL of blood collected using finger-prick method and a Mitra® device. Assay was validated with satisfactory results obtained for each tested parameter and demonstrated for all 27 compounds: LLOQ at the level 0.1 ng/ml or 0.5 ng/ml, good linearity in the range 0.1/0.5 – 50 ng/ml (regression coefficient in the range: 0.997 – 0.999), %CV – 3-13% and intermediate precision in the range of 93-113%, depending on the tested compound. Obtained data showed that VAMS coupled with optimized targeted LC-MS/MS analysis can be successfully applied for selected psychoactive compounds determination. Novel aspect:New testing procedure using VAMS and LC-MS/MS for quantitative determination of 27 psychoactive compounds. |
 ASMS 2022, Minneapolis, USA, June 5 – 9, 2023. ASMS 2022, Minneapolis, USA, June 5 – 9, 2023.
|