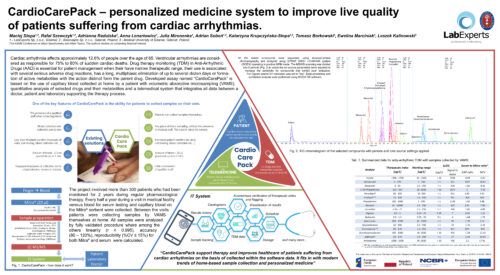
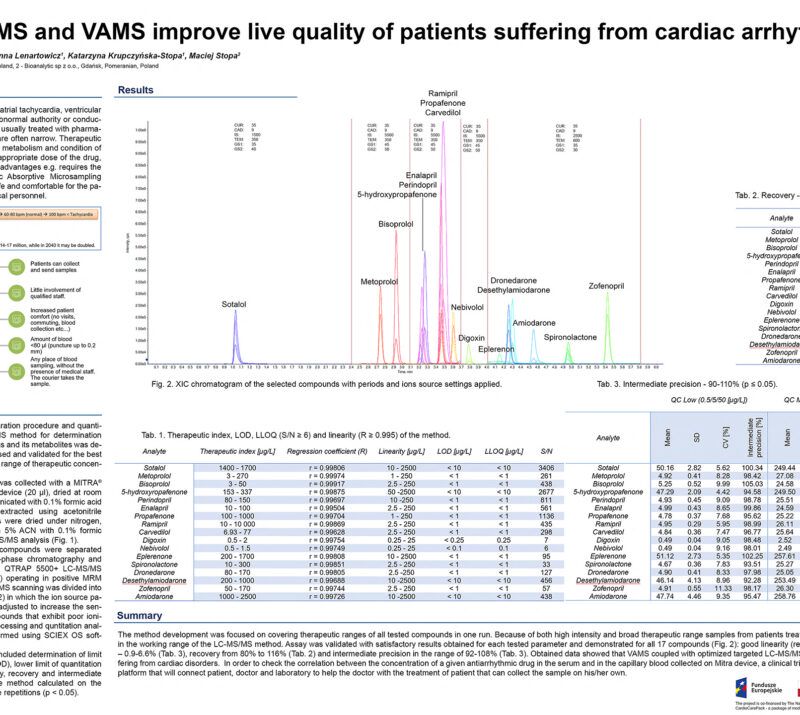
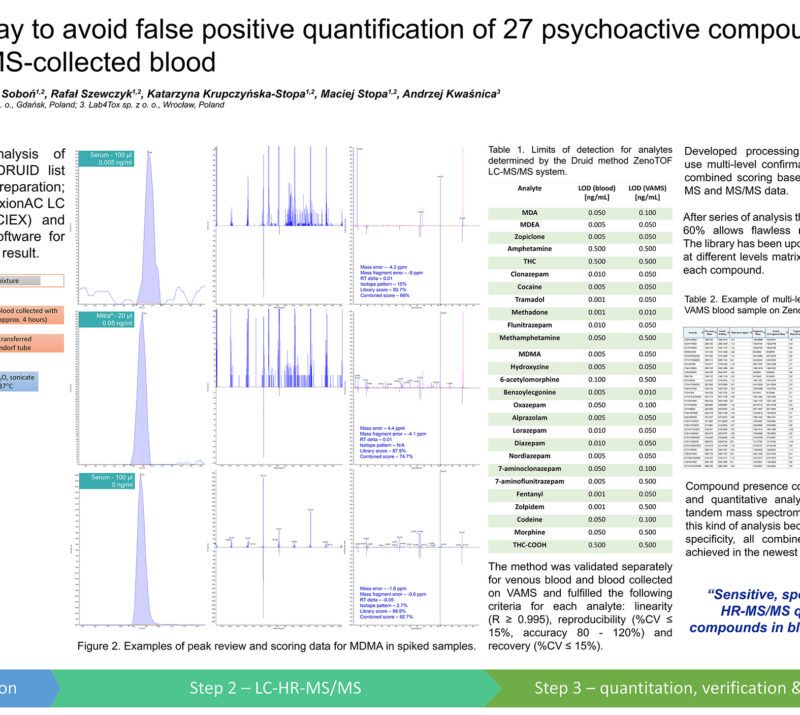
CardioCarePack – personalized medicine system to improve live quality of patients suffering from cardiac arrhythmias.Maciej Stopa1, 2; Rafał Szewczyk1, 2; Adrianna Radulska3, Anna Lenartowicz2; Julia Mironenka2; Adrian Soboń1, 2; Katarzyna Krupczyńska-Stopa1, 2; Tomasz Borkowski3; Ewelina Marciniak3, Leszek Kalinowski3 1Bioanalytic Sp. z o.o., Gdansk, Poland; 2LabExperts Sp. z o.o., Gdansk, Poland; 3 Medical University of Gdansk, Gdansk, Poland AbstractCardiac arrhythmia affects approximately 12.6% of people over the age of 65. Ventricular arrhythmias are considered as responsible for 75% to 80% of sudden cardiac deaths. Therapy of the disease include ablation, use of cardioverter or defibrillator and pharmacological treatment where the key for successful therapy lays in maintaining the necessary concentration of drugs. The goal can be achieved by therapeutic drug monitoring (TDM). Developed assay named “CardioCarePack” is based on the use of capillary blood collected and dried at home by a patient with volumetric absorptive microsampling (VAMS), quantitative analysis of selected drugs and their metabolites and a telemedical system that integrates all data between a doctor, patient and laboratory supporting the therapy process. CardioCarePack is ready for commercialization. Methods:Blood collected with a MITRA® microsampling device (20 µl) is prepared with full validated protocol that include sonication with 0.1% formic acid in water followed by acetonitrile extraction, evaporation under nitrogen stream and resuspension in LC mobile phase. Sample is subjected to LC-MS/MS analysis in positive ionization MRM mode with QTRAP 5500+ mass spectrometer (SCIEX) or similar one. Analysis covers simultaneous quantitation of 15 drugs: Sotalol, Metoprolol, Bisoprolol, Propafenone, Carvedilol, Nebivolol, Amiodarone, Digoxin, Perindopril, Ramipril, Eplerenone, Spironolactone, Zofenopril and 2 metabolites: 5-hydroxypropafenone and Desethylamiodarone. Results are placed in a server-based telemedical system where depending on accession rights history, doses, therapeutic index flagging, cardiograms, sample tracking and many other data are available for patient, doctor and laboratory staff, respectively. Preliminary Data:The project involved more than 300 patients who had been monitored for 2 years during regular pharmacological therapy. Every half a year during a visit in medical facility venous blood for serum testing and capillary blood on the MITRA® sorbent were collected. Between the visits patients were collecting samples by VAMS themselves at home. All samples were analyzed by fully validated procedure where among the others linearity (r ≥ 0.995), accuracy (80 – 120%), reproducibility (%CV ≤ 15%) for both MITRA® and serum samples were calculated. The LC-MS/MS method developed for 17 compounds covers therapeutic range of the tested drugs: 0.25 – 25 ng/ml (Digoxin and Nebivolol) 2.5 – 250 ng/ml (Metoprolol, Bisoprolol, Propafenone, Carvedilol, Perindopril, Ramipril, Spironolactone, Zofenopril and 25 – 2500 ng/ml (Sotalol, Desethylamiodarone, Eplerenone, Amiodarone, 5-hydroxypropafenone). Results generated during LC-MS/MS analysis were analyzed with SCIEX OS 1.7 software. On the basis of samples collected during 4 visits in medical facility a correlation between drugs concentration in venous blood (serum) and capillary blood (MITRA®) (S/M) was calculated. For some compounds S/M ratio was close to 1 (ex. Sotalol – 0.95, %CV – 5.12%, Nebivolol – 1.03, %CV – 3.17%) or moderately to more than 2-fold different (ex. Digoxin – 0.69, %CV – 1.46%, Ramipril – 1.18, %CV – 11.1, Perindopril – 1.86, %CV – 4.73%, Amiodarone – 2.17, %CV – 10.78). The correlation factors are statistically significant (p < 0.05) and can be used for accurate concentration estimation in venous blood. Developed telemedicine system integrate CardioCarePack service – a patient, doctor and LC-MS/MS laboratory. The system helps in Doctor’s supervision over the patient’s condition on the basis of collected within the system data ex.: autonomous verification of the therapeutic index with flagging, visualization of results, tracking samples on the patient-doctor line, schedule of visits, results history and many more. Novel aspect:A modern telemedicine system for TDM of cardiological drugs based on LC-MS/MS analysis of samples collected at home with VAMS. |
ASMS 2023, Houston, USA, June 4 – 8, 2023 . |