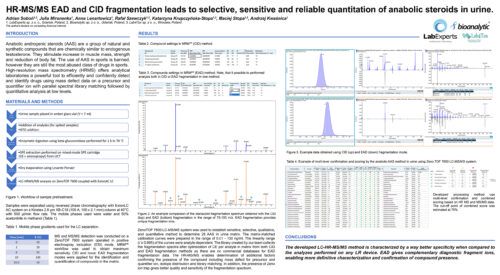
HR-MS/MS EAD and CID fragmentation leads to selective, sensitive and reliable quantitation of anabolic steroids in urine.Adrian Soboń (1,2); Julia Mironenka (1); Anna Lenartowicz (1); Rafał Szewczyk (1,2); Katarzyna Krupczyńska-Stopa (1,2); Maciej Stopa (1,2); Andrzej Kwaśnica (3) AbstractIntroductionAnabolic androgenic steroids (AAS) are a group of natural and synthetic compounds that are chemically similar to and mimic the actions of endogenous testosterone. They stimulate androgen receptors, contributing to an increase in muscle mass and strength, and on the other hand reduction of body fat. Although the use of AAS in sports is banned, they are still the most abused class of drugs in sports. High-resolution mass spectrometry (HRMS) offers analytical laboratories a powerful tool to detect drugs and efficiently and confidently identify them using mass defect data on precursor and quantifier ion with parallel spectral library matching followed by quantitative analysis at low levels. Methods:Sample preparation starts with enzymatic hydrolysis of potential anabolic conjugates, followed by the clean-up procedure using a mix-mode SPE cartridge (C8 + aminopropyl). AAS were analyzed using ZenoTOF 7600 system with the time scheduled Zeno MRMhr for optimal sensitivity using both collision-induced dissociation (CID) and electron-activated dissociation (EAD). Chromatographic separation was done on a Kinetex 2.6 µm XB-C18 (100 A; 100 x 2.1 mm) column using an ExionLC AC system (Sciex). Mobile phase solvents were water (A) and mix of methanol and acetonitrile (1:1 v/v) (B). Separation was performed at a flow rate of 500 µl/min with a column temperature of 40 °C. Data processing was made using SCIEX OS v.3.1 software. Preliminary Data:The gold standard in the LC-MS/MS quantitation is the scanning of two MRM pairs on low resolution (LR) triple quad instrument – quantifier and qualifier ion. Unfortunately, matrix effects may lead to insufficient confirmation of compound presence, especially at low levels where MRM ratio and matrix interferences can lead to result misinterpretation. To overcome the specificity problems in the LR data, HR system was used to establish sensitive, selective, qualitative and quantitative method to determine 28 AAS in urine matrix. Both collision-induced dissociation (CID) and electron-activated dissociation (EAD) fragmentation methods were used to select specific fragment ions for quantitation and MS/MS libraries for analytes. The matrix-matched calibration curves was prepared in the range of 0.01 – 100 ng/ml. The linearity range (r ≥ 0.995) of the curves were analyte dependent. Lower limits of quantification can be achieved for some compounds. EAD is an alternative fragmentation mode that produces unique fragmentation ions, which can be used as diagnostic ions for compound differentiation. The creation of our own library was important because in the developed method MS/MS spectrum is collected for the optimized collision energy (CE), while the spectra deposited in commercial databases are generated at a constant CE value and contain only a few of the tested substances. Moreover, there are no commercial databases for EAD fragmentation data. The created LC-MS/MS method is characterized not only by high sensitivity, but by using a HR-MS/MS, it enables determination of additional factors confirming the presence of the compound including mass defect for precursor and quantifier ion, isotope distribution and finally library matching. In addition, the presence of Zeno ion trap gives better quality and sensitivity of the fragmentation spectrum. The developed LC-HR-MS/MS method is characterized by a much better specificity compared to the analyzes performed on any LR device. Novel aspect:Sensitive and selective LC-HR-MS/MS method using CID and EAD fragmentation modes for anabolic steroids quantitation in human urine with confidence. |
 ASMS 2023, Houston, USA, June 4 – 8, 2023. ASMS 2023, Houston, USA, June 4 – 8, 2023.
|