
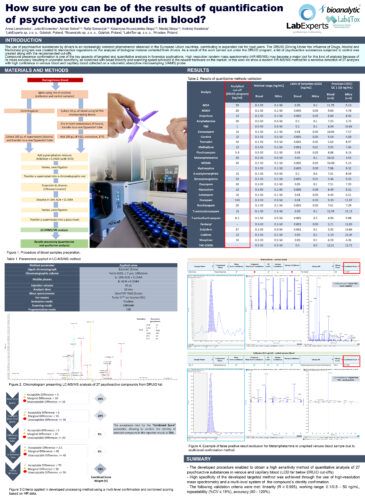
How sure you can be of the results of quantification of psychoactive compounds in blood?Anna Lenartowicz (1); Julia Mironenka (1); Adrian Soboń (1,2); Rafał Szewczyk (1,2); Katarzyna Krupczyńska-Stopa (1,2); Maciej Stopa (1,2); Andrzej Kwaśnica (3) AbstractIntroductionCompound presence confirmation is one of the key aspects of targeted and quantitative analysis in forensics applications. High resolution tandem mass spectrometry (HR-MS/MS) may become a major tool for this kind of analysis because of its mass accuracy resulting in unparallel specificity, all combined with broad linearity and scanning speed achieved in the newest hardware on the market. In this work we show a modern HR-MS/MS method for a sensitive detection of 27 analytes with high confidence in venous blood and blood collected on a volumetric absorptive microsampling (VAMS) probe. Analytes include: Tramadol, Morphine, Fentanyl, Methadone, Codeine, Alprazolam, Diazepam, Flunitrazepam, Lorazepam, Oxazepam, Zolpiclone, Clonazepam, Nordiazepam, Zolpidem, Hydroxyzine, Amphetamine, Methamphetamine, MDA, MDMA, MDEA, THC, Cocaine, 6-acetylmorphine, 7-aminoflunitrazepam, 7-aminoclonazepam, Benzoylecgonine, THC-COOH. Materials and MethodsBriefly, sample preparation included: spiking of internal standards, protein precipitation and extraction using acetonitrile:methanol (1:1) with 0.1% formic acid, evaporation under nitrogen stream and reconstitution in LC mobile phase. Compounds were analyzed using reversed-phase chromatography on ExionAC LC coupled with ZenoTOF 7600 (SCIEX) operating in positive electrospray ionization scheduled MRMhr mode acquiring full MS/MS spectra for each compound. Developed processing method use multi-level confirmation and combined scoring based on HR data where result is affected by 5 criteria that have different decision weights: MS/MS library confirmation (50%), precursor ion mass defect (20%), quantifier ion mass defect (20%), precursor ion isotope pattern (5%) and retention time (5%). Each criterium contributes in the combined score calculation and has its own range of 3-level acceptance in ppm, % or seconds, respectively. The cut-off point of combined score is estimated at 70% after series of analysis and allows flawless reporting of compounds from DRUID list. Data were processed in SCIEX OS software. ResultsThe method was validated separately for blood and blood collected on VAMS and fulfilled the following criteria for each analyte: linearity (R ≥ 0.995), working range (LLOQ – 50 ng/ml), reproducibility (%CV ≤ 15%), accuracy (80 – 120%) and recovery (80 – 120%). For majority of analytes included in the method LLOQ was in the range 0.01- 0.05 ng/ml (ex. Diazepam, Flunitrazepam, Kodeine, Morphine, MDA) for blood and 0.05-0.1 ng/ml for VAMS sampler. For analytes such as Tramadol, Fentanyl, Methadone and Cocaine the LLOQ ranged between 0.001- 0.005 ng/ml for blood and 0.01-0.05 for VAMS. The method was successfully applied to routine analysis of blood samples. Discussions and ConclusionsA quantitative LC-MS/MS method was validated according to the international recommendations. The greatest advantage of presented method is its high specificity and extremely low Limits of Quantitation. Most of described in literature LC-MS/MS methods used for psychoactive compounds quantification are based on MRM scanning mode with triple quadrupole instruments. Such approach does not allow to obtain sufficiently high specificity because of difficult data interpretation at low concentrations of analytes in real-life samples where MRM ratio and matrix interferences leads to data misinterpretation. Developed method allows sensitive, specific and false positive resistant HR-MS/MS quantitation of 27 psychoactive compounds in blood and blood collected by VAMS. |
 IATDMCT 2023, Oslo, Norwegia, 24—27.09.2023. |


